
Professor Lu Liangqiu of Central China Normal University (click to view introduction) has long been engaged in the research of transition metal-catalyzed cyclization reactions, and has accumulated rich experience in efficient and precise synthesis of heterocycles (Chem. Soc. Rev. 2022, 51, 4146-4174; Angew. Chem. Int. Ed. 2023, 62, e202212444; Angew. Chem. Int. Ed. 2022, 61, e202117215; J. Am. Chem. Soc. 2022, 144, 19932-19941)。 The team used palladium- and iron-catalyzed palladium to achieve [2014+2016] cycloaddition of vinylbenzoxazinone with thionelid in 4 and 1 (Nat. Commun. 2014, 5, 5500-5509; Angew. Chem. Int. Ed. 2016, 55, 2840-2844)。 In addition, in 2016, the team also developed an ethynylbenzoxazinone reagent to achieve a copper-catalyzed asymmetric [4+1] cycloaddition reaction (J. Am. Chem. Soc. 2016, 138, 8360)。 These cycloaddition reactions all utilize metal-polarized nitrogen-o-methylenequinone intermediates, but their benzene rings themselves do not participate in the reaction. Recently, the authors have discovered a new mode of reaction for this type of active intermediate—a universal transient aromatial-rearomatization process (Figure 1c). This kind of reaction first produces spiroazetidine (aoQSAs) intermediates through the benzene ring aromatization process, and then produces benzimidazoline and dibenzodiazepine nitrogen heterocyclic compounds according to the different benzyl functional groups (FG) and then through different aromatization processes. Experiments show that the reaction generally has a wide range of substrate applications, and can be compatible with substituents and functional groups with different electrical and steric steric hindrance.
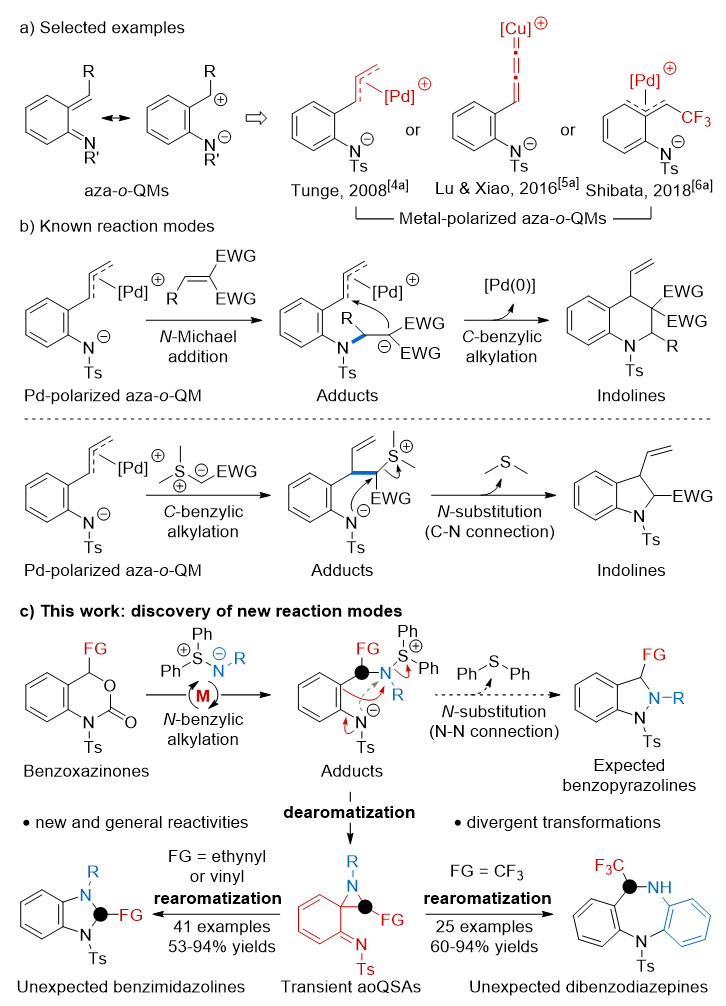
Figure 1. New reactivity studies of metal-polarized nitrogeno-oquinones
At the same time, the authors propose a possible reaction mechanism based on the available literature and experimental results (Figure 2). First, benzoxazinone substrates react with copper or palladium catalysts to produce metal-polarized nitrogen-o-methylenequinones C or G. Next, C or G undergoes a benzylation reaction with azetiphatilide 2a. The newly formed zwitterionic intermediates E or J produce similar tension ring intermediates K or L by the aromatic spirozocyclic propaneation process. Finally, the differentiated aromatization process yields benzimidazoline or dibenzodiazepine azoxacyclonic compounds. To verify this mechanism, the research team used LC-MS and 19The F NMR technique verified the generation of intermediates E and J, and used LC-MS/MS to analyze retention time, MS and its fragmentation peak to detect the presence of spirozocyclopropane active intermediate L.
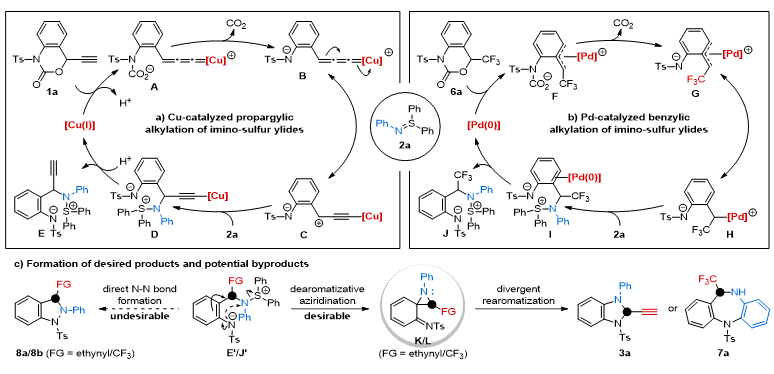
Figure 2. Mechanism of copper and palladium-catalyzed aromatial decomposition-recovery aromatic tandem cyclization
The research team, in collaboration with Associate Professor Rao Li of Central China Normal University, performed an energy analysis of the six possible pathways that the reaction could travel through DFT theory calculations (Figure 3). The results show that the direct N-N reaction of intermediates E' or J' overcomes a higher energy barrier than the generation of sprazazidine intermediates (aoQSAs). To elucidate the effects of ethynyl and trifluoromethyl groups on the two aromatization processes, the research team analyzed Path III (protocol 3a, shown by the blue dashed line) and path VI (protocol 3b, shown by the red dashed line) by DFT calculation. The results showed that the two pathways needed to overcome higher energy barriers, 7.3 kcal/mol and 4.0 kcal/mol, respectively (TS2A vs TS2B; TS4B vs TS4A)。 These results explain the delocalization effect caused by the acetylene group to promote the formation of imine ions in intermediate M, and why the product is not diastereoselective when using chiral ligands. In contrast, electron-deficient trifluoromethyl groups had the opposite effect (ethynyl: 0.4 kcal/mol; Trifluoromethyl: 11.6 kcal/mol), thus opting for the Cope rearrangement pathway, resulting in a different heterocyclic backbone.

Figure 3. DFT theoretical calculations
The research team also collaborated with Professor Tan Ying of Shenzhen International Graduate School of Tsinghua University to study the antitumor activity of dibenzodiazepines in vitro using cell phenotypic screening technology, and discovered a heterocyclic molecule 7y with development potential. Since then, the authors have conducted a series of biological experiments on compound 7y and found that it exhibits broad-spectrum antiproliferative activity in a variety of cancer cell lines (see Table 1). Anti-tumor evaluation in the A431 cell line showed that compound 7y was able to inhibit the proliferation and metastasis of tumor cells, induce apoptosis and cell cycle arrest, and exhibit good antitumor activity. These results suggest that the compound has the potential to be further developed as an anti-tumor drug candidate.

Table 1. Antiproliferative activity of compound 7y in tumor cell lines
The results were recently published in Angewandte Chemie International Edition, and the first authors of the paper are Wang Baocheng (experimental part), associate professor Rao Li (computing part), and Fang Kaixin, a master's student at Tsinghua University Shenzhen International Graduate School (Bioactive part), Professor Lu Liangqiu and Professor Tan Ying are co-corresponding authors of the paper.
Name: Eric Yuan
Mobile:86-18427358861
Tel:86-18427358861
Whatsapp:+86 18427358861
Email:yihekeji@chembuying.com
Add: