
Recently, Duan Weiliang's team (click to view introduction) reported copper-catalyzed asymmetric C (sp2–H bond arylation reaction. The synthesis method has the advantages of wide application range of substrates, high enantioselectivity, low economic cost, and high application prospects of the corresponding products, which provides a new way for the application of cheap metal copper in asymmetric carbon-hydrogen bond activation reactions.
The team used chiral bisoxazolin with a rigid skeleton as a chiral ligand and diaryl high iodized salt as a functional mass reagent, which was achieved for the first timePhosphorus-oxygen-guided copper-catalyzed ortho-C(sp2−H bond asymmetric arylation reaction to synthesize a series of phosphorus chiral phosphoryldiamine compounds and axial chiral aryl phosphooxides with up to 92% enantioselectivity(Figure 1).
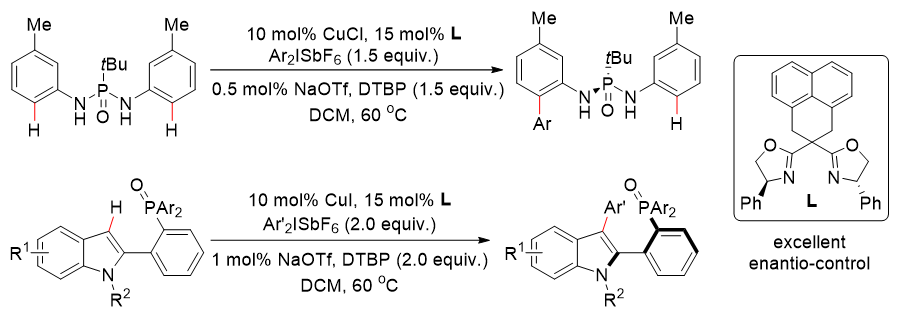
Figure 1. Copper-catalyzed asymmetric C(sp2−H bond arylation to synthesize phosphorus chiral and axial chiral phosphorus-containing compounds. Photo by Nat. Commun
In the course of the experiment, the authors found that trace amounts of sodium trifluoromethanesulfonate additive played a key role in the smooth progress of the above reaction, and believed that the trifluoromethanesulfonate anion could assist hydrogen extraction during the carbon-hydrogen bond activation of the reaction. In addition, the reaction is sensitive to the electron effect of the substrate, and the substrate replaced by the electron functional group can be smoothly arylated, and the electron-donor or pull-electron substituents of diaryl high iodized salt have a certain influence on the reaction results.
Based on the above experimental results, combined with intramolecular competition experiments, kinetic isotope effect experiments and other mechanism research experiments, the authors speculate that trivalent aryl copper species III with electrophilic properties is the key active intermediate of the reaction, which can electrophilically activate the electron-rich aromatic ring of the substrate under the control of the ligand, so as to realize the enantioselective copper-catalyzed carbon-hydrogen bond arylation reaction, the specific catalytic cycle process is shown in Figure 2.

Figure 2. Possible catalytic cyclic processes. Photo by Nat. Commun
It is worth mentioning that the team carried out gram-scale scale-up experiments on the copper-catalyzed carbon-hydrogen bond arylation reaction they developed, and found that the reaction can still proceed smoothly under low catalyst load conditions, indicating that the above copper-catalyzed system may have potential cost advantages.
The above research results have been published in Nature Communications, with Yan Shaobai, a doctoral student at Yangzhou University, as the first author and Professor Duan Weiliang as the corresponding author.
Name: Eric Yuan
Mobile:86-18427358861
Tel:86-18427358861
Whatsapp:+86 18427358861
Email:yihekeji@chembuying.com
Add: