Institute of Organic Research: Asymmetric total synthesis of Phomarol, a rearranged steroidal natural product based on biomimetic SN2′ cyclization
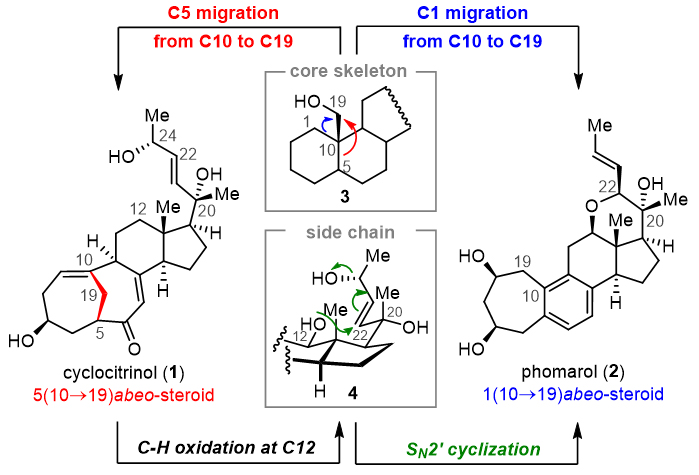
Steroidal natural products have received extensive attention from organic chemists because of their novel skeleton and important biological activity. Gui Jinghan, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences (click to view introduction), used biomimetic synthesis strategies to complete the efficient synthesis of multiple rearranged steroidal natural products such as cyclocitrinols, propindilactone G, pinnigorgiols, sarocladione and bufospirostenin A. Recently, the team made a new breakthrough in the synthesis of rearranged steroid natural products, and completed the asymmetric total synthesis of 1 (10→19) migratory steroid phomarol in a 13-step reaction concisely and efficiently, and the relevant results were published in the Journal of the American Chemical Society (J. Am. Chem. Soc.)。