yihekeji@chembuying.com
86-18427358861

| 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde Basic information |
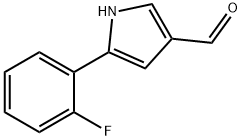
| Product Name: | 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde |
| Synonyms: | 1H-Pyrrole-3-carboxaldehyde, 5-(2-fluorophenyl)-;5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde;5-(2-Fluorophenyl)-1H-pyrrole-3-carbal...;5-(2-Fluorophenyl)-1H-pyrrole-3-carboxaldehyde;1H-Pyrrole-3-carboxaldehyde, 5-(2-fluorophenyl)-1H-pyrrole-;TAK438 Impurity 54;1H-Pyrrole-3-carboxaldehyde, 5-(2-fluorophenyl)-1H-pyrrole-3-carbaldehyde;TAK-438( Vonoprazan fumarate) intermediate 3 |
| CAS: | 881674-56-2 |
| MF: | C11H8FNO |
| MW: | 189.19 |
| EINECS: | 809-913-6 |
| Product Categories: | Vonoprazan;Pharmaceutical Intermediates;881674-56-2 |
| Mol File: | 881674-56-2.mol |
|
|
| 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde Chemical Properties |
| Melting point | 133 °C |
| Boiling point | 370.6±32.0 °C(Predicted) |
| density | 1.270±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Chloroform (Slightly, Sonicated), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 14.77±0.50(Predicted) |
| color | Light Brown to Brown |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C11H8FNO/c12-10-4-2-1-3-9(10)11-5-8(7-14)6-13-11/h1-7,13H |
| InChIKey | MQULPEUCGKEHEG-UHFFFAOYSA-N |
| SMILES | N1C(C2=CC=CC=C2F)=CC(C=O)=C1 |
| Safety Information |
| HS Code | 2933998090 |
|
|
| MSDS Information |
| 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde Usage And Synthesis |
| Chemical Properties | Light orange to Yellow to Green powder to crystal. |
| Uses | 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde acts as a reagent in the synthetic preparation of novel pyrrole derivatives as potassium-competitive acid blocker (P-CAB). |
| Application | 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde is a potassium ion-competitive acid blocker used in therapy Acid-related diseases, gastric ulcer, duodenal ulcer, reflux esophagitis, etc. |
| Preparation | Synthesis of 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde: Using pyrrole as raw material, after being protected by N-alkylation of triisopropylsilyl chloride, it is then reacted with Vilsmeier reagent to obtain 1H-Pyrrole-3-carbaldehyde, which is then purified by N-bromosuccinimide (NBS) bromination and 2-fluorophenylboronic acid for Suzuki coupling reaction to obtain crude 5-(2-Fluorophenyl)-1H-pyrrole-3-carbaldehyde, which was purified with toluene. The total yield was 35.5%. |
Name: Eric Yuan
Mobile:86-18427358861
Tel:86-18427358861
Whatsapp:+86 18427358861
Email:yihekeji@chembuying.com
Add: